Can Gram Staining Reagents Be Stored in a Refrigerator?
News 16 12 月, 2025
Recently, some technically curious customers have raised questions about the storage conditions of microbiological testing reagents. Many reagents are labeled for storage at 2–8 °C, while others are recommended for 2–25 °C in a cool, dark place.This leads to a common question: Why are Gram staining reagents usually stored at room temperature rather than in…
Causes of Condensation (“Water Droplets”) on Slant Cultures and Preventive Measures
News 16 12 月, 2025
Core Cause: Metabolic Heat Generation and Temperature Fluctuations Condensation on the surface of agar slants during incubation is a common phenomenon. The primary cause is the interaction between microbial metabolic heat and temperature changes within the culture tube. 1. Active Growth Phase: Heat Production and Moisture Accumulation During t…
Why Are Agar Plates Incubated Upside Down After Inoculation?
News 16 12 月, 2025
Many laboratory beginners ask the same question: Why are agar plates inverted during incubation?Inverting culture plates is a standard microbiological practice based on contamination control, colony quality, and operational safety. The reasons are explained below. I. Reasons for Inverting Plates Before and During Incubation 1. Prevention of Conde…
Troubleshooting No Colony Formation on Agar Plates
News 16 12 月, 2025
A Comprehensive Analysis of Strains, Techniques, and Culture Media Have you ever prepared culture media late into the night, inoculated your samples carefully, only to find completely blank agar plates the next day? Even when protocols are followed precisely, microorganisms may fail to grow. This article provides a systematic approach to identifying the…
What to Do If a Mold Incubator Shows Growth in a Blank Control?
News 10 12 月, 2025
If a mold incubator shows microbial growth in a blank control (i.e., mold or contaminants appear in an uninoculated dish), the core cause is residual contamination inside the incubator, improper aseptic technique, contaminated consumables, or secondary contamination from the surrounding air.Below is a prioritized troubleshooting guide including root causes…
The Role of HEPES Buffer in Cell Culture Media
News 10 12 月, 2025
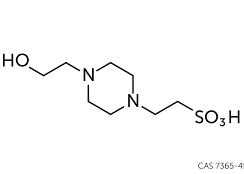
When should HEPES be used in cell culture media? HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is a commonly used zwitterionic buffer with a pKa of 7.3 at 37°C. In addition to sodium bicarbonate, HEPES can be incorporated into culture media because it is important to maintain sufficient bicarbonate for nutritional purposes. When necessary, …
Glutamine in Cell Culture
News 14 10 月, 2025
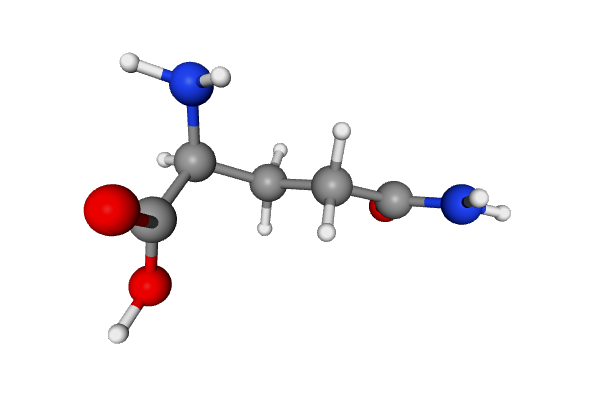
L-Glutamine, a coded amino acid in protein synthesis, is a common serum-free culture medium supplement used to support the proliferation and physiological functions of various mammalian cells. In commonly used culture media, glutamine concentrations range from 0.5 mM in Ames medium to 10 mM in MCDB 131 medium, typically between 2–4 mM. Primary Funct…
Causes and Solutions for Cell Vacuolization
News 14 10 月, 2025
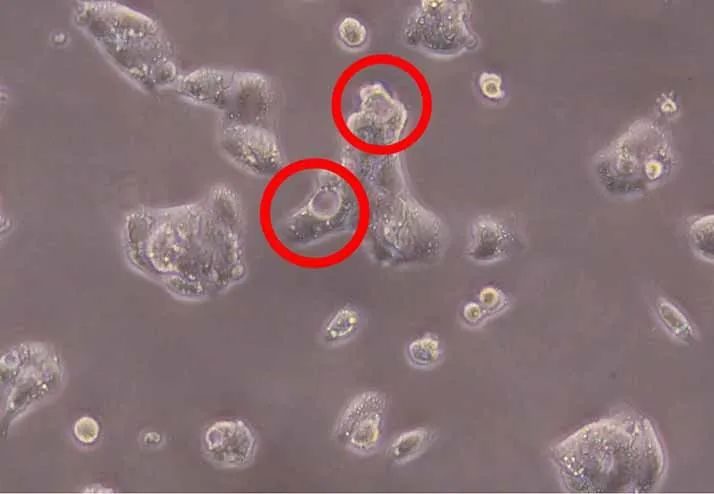
During cell culture, researchers may occasionally observe vacuoles forming within cells. Vacuoles are bubble-like structures in the cytoplasm that differ in appearance from the surrounding cell content. This article explains the main causes of cell vacuolization and provides guidance on how to prevent and resolve the issue. Definition Cell vacuolizat…
Preventing and Handling Black Dots in Cell Culture
News 14 10 月, 2025
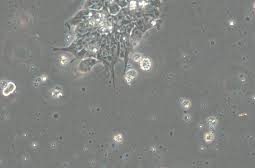
When culturing cells, many researchers notice small black dots under the microscope and wonder — are they cell debris or contamination? How to Identify the Cause 1. Observe movement:Particles showing slow Brownian motion are usually harmless cell debris, while rapidly moving dots may indicate bacterial contamination. 2. Check the medium:Contaminat…
Key Principles of Aseptic Filling Monitoring in Cleanrooms
News 8 9 月, 2025
In sterile pharmaceutical manufacturing, aseptic filling is one of the most critical processes. Since injectable drugs bypass the body’s natural immune defenses, even the smallest contamination during filling can put patients at risk. To ensure safety, manufacturers must operate in cleanrooms that meet the strictest global standards and maintain rigorous m…
Preventing Culture Medium Contamination: Key Principles for Reliable Laboratory Testing
News 8 9 月, 2025
In clinical diagnostics, vaccine development, biopharmaceuticals, and biomedical research, culture media serve as the foundation for microbial isolation, antibiotic susceptibility testing, cell modeling, and pathogenicity studies. The quality of culture media directly affects pathogen detection, experimental accuracy, and production safety. Even a single f…
Microbial Detection in Food Safety: Plate Count Method vs. Flow Cytometry
News 18 8 月, 2025
Food safety relies heavily on microbial detection, which plays a critical role in assessing contamination levels, identifying hazards, and ensuring compliance with testing requirements. Selecting the right detection method depends on the type of microorganism, the food matrix, and the goals of the analysis. Among various approaches—including traditional…